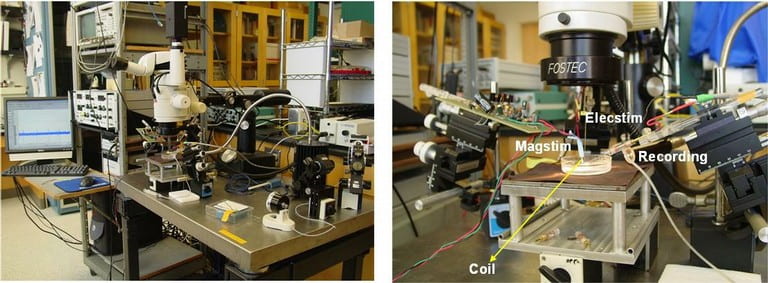
Neural stimulation is commonly accomplished by a voltage or current pulse through a microelectrode. Ideally, a method would exist which inherently had zero net charge transfer, required only simple driver circuitry and was completely isolated from the tissue to reduce circuit failure due to corrosion and fouling by protein deposition. Magnetic stimulation achieves these goals. The presence of scar tissue or deposited proteins is irrelevant because the magnetic permeability of tissue is near that of free space. Excitation arises from the magnetic field which generates a current across the membrane of the cell which changes the resting potential of the neuron and triggers an action potential (the fundamental signal generation mechanism neurons employ). We are currently studying the fundamental mechanisms of magnetic stimulation, developing models and verifying through in vitro experiments.

Selected Publications
- “Functional Magnetic Stimulation for Epiretinal Prosthesis”, E. Basham, W. Liu, and M. Sivaprakasam, Abstract B254, Association of Research in Vision and Ophthalmology Annual Meeting, May 2005.
Collaborators
- Santa Clara University