Non-Invasive Functional Magnetic Stimulation
Neural stimulation is commonly accomplished by a voltage or current pulse through a microelectrode. Ideally, a method would exist which inherently had zero net charge transfer, required only simple driver circuitry and was completely isolated from the tissue to reduce circuit failure due to corrosion and fouling by protein deposition. Magnetic stimulation achieves these goals. The presence of scar tissue or deposited proteins is irrelevant because the magnetic permeability of tissue is near that of free space. Excitation arises from the magnetic field which generates a current across the membrane of the cell which changes the resting potential of the neuron and triggers an action potential (the fundamental signal generation mechanism neurons employ). We are currently studying the fundamental mechanisms of magnetic stimulation, developing models and verifying through in vitro experiments.

Selected Publications
- “Functional Magnetic Stimulation for Epiretinal Prosthesis”, E. Basham, W. Liu, and M. Sivaprakasam, Abstract B254, Association of Research in Vision and Ophthalmology Annual Meeting, May 2005.
Collaborators
- Santa Clara University
Microstimulation Platform for Neural Systems
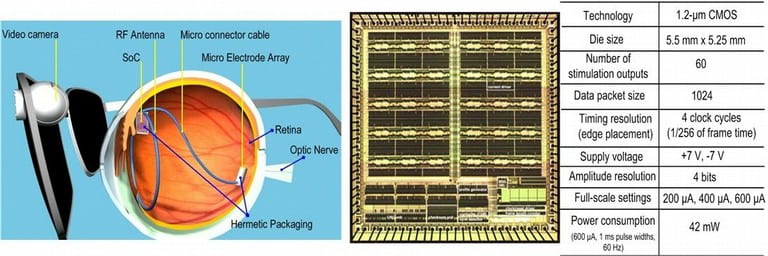
Functional Electrical Stimulation of biological tissue has a wide range of applications ranging from pain relief to neural prostheses. Flexibility, small size and low power operation, safety are key requirements in microstimulation systems. Custom integrated circuits for microstimulation face the challenge of having to support relatively high stimulation voltages for the current CMOS technology, while still needing maintain low power operation and achieve a high degree of miniaturization. In addition, the experimental nature of the evolving microstimulation applications demands a high degree of flexibility and versatility. We are working on several microstimulation applications through our collaborators that have a varying degree of requirements.

Selected Publications
- “Challenges for Integrated Circuits in Implantable Devices,” W. Liu and M. Sivaprakasam, Volume: 20, Future Fab International, January 2006.
- “Microelectronics Design for Implantable Wireless Biomimetic Microelectronic Systems,” W. Liu, M. Sivaprakasam, G. Wang, M. Zhou, J. Granacki, J. LaCoss, and J. Wills, Volume: 24, Pages: 66 – 74, IEEE Engineering in Medicine and Biology Magazine, September 2005.
- “A Variable Range Bi-Phasic Current Stimulus Driver Circuitry for an Implantable Retinal Prosthetic Device,” M. Sivaprakasam, W. Liu, M. S. Humayun, and J. D. Weiland, Volume: 41, Pages: 763 – 771, IEEE Journal of Solid State Circuits, March 2005.
Collaborators
- University of Southern California
- Long Beach Veteran Affairs
- Stanford University
- Huntington Medical Research Institutes
Next Generation Retinal Prosthesis
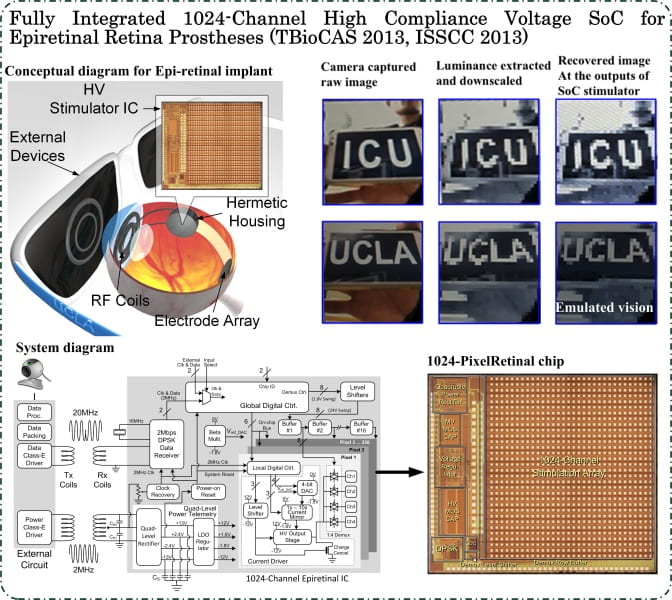
We continue our pioneering work on Retinal Prosthesis to restore vision in blind patients with Retinitis Pigmentosa (RP) and Age-related Macular Degeneration (AMD) through developing the next generation retinal implants. The goal of these implants is more than 1000 pixels that would enable facial recognition and independent mobility. The project focuses on delivering power and data to the retinal implant inside the eye and the implant microstimulator electronics which delivers the current pulses to stimulate the retinal layer to elicit visual perception. Since the use of invasive means such as tethering wires results in discomfort and potential infection, a completely wireless approach is used to transfer both power and data. Since the coupling between the external unit consisting of the power transmitter and the power receiver can vary due to the patient’s movements, a closed loop approach is used which varies the transmitted power dynamically to automatically compensate for such movements. We are collaborating with the medical team in University of Southern California and several national laboratories for this project.

Selected Publications
- “Retinal Prosthesis,” J. D. Weiland, W. Liu, and M. S. Humayun, Volume: 7, Pages: 361 – 401, Annual Review of Biomedical Engineering, August 2005.
- “A Core Component for Neuro Stimulus with Telemetry Unit,” W. Liu, M. Humayun, K. Vichienchom, M. Clements, S. DeMarco, E. McGucken, C. Hughes, E. de Juan, J. Weiland, R. Greenberg, Volume: 35, Pages: 1487-1497, IEEE Journal of Solid-State Circuits, October 2000.
Collaborators
- University of Southern California
- California Institute of Technology
- Lawrence Livermore National Laboratory
- Sandia National Laboratory
- Argonne National Laboratory
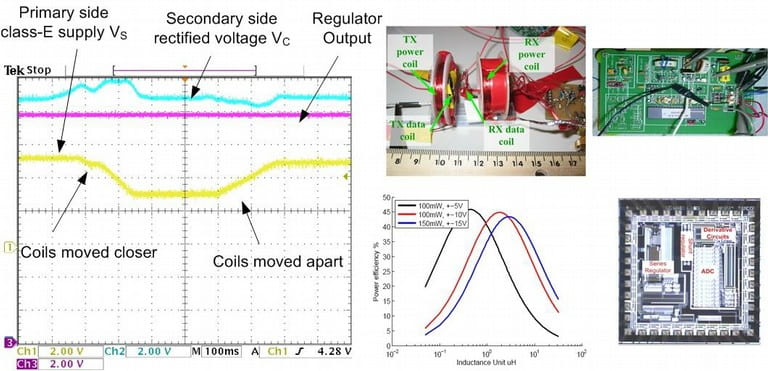
Power and Data Telemetry
Power and Data Telemetry for Biomimetic Microelectronic Systems
The newly developed systems from NSF BMES ERC will allow bi-directional communication with tissue and by doing so enable implantable/portable microelectronic devices to treat presently incurable diseases such as blindness, paralysis, and certain central nervous system disorders. Our role in the center’s goal is guided by four basic themes and fundamental challenges: power efficiency, bi-directional communication capability, miniaturization, and integration. We are addressing the above challenges through fundamental theory and prototyping of subsystems such as wireless power and data telemetry, microstimulators, neural recording systems, image processing.

Selected Publications
- “Optimization of Coils for Biomedical Applications,” Z. Yang, W. Liu, and E. Basham, IEEE Transactions on Magnetics, Volume: 43, Pages: 3851 – 3860, October 2007.
- “A Non-Coherent PSK Receiver with Interference Canceling for Transcutaneous Neural Implants,” M. Zhou and W. Liu, International Solid-State Circuits Conference, February 2007.
- “Design and Analysis of an Adaptive Transcutaneous Power Telemetry for Biomedical Implants,” G. Wang, W. Liu, M. Sivaprakasam, and G. A. Kendir, IEEE Transactions on Circuits and Systems – I, Volume: 52, Pages: 2109 – 2117, October 2005.
Collaborators
- University of Southern California
- California Institute of Technology