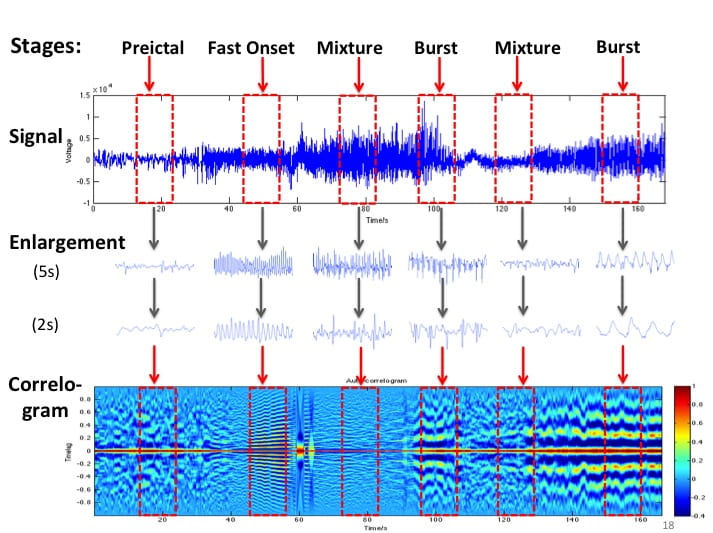
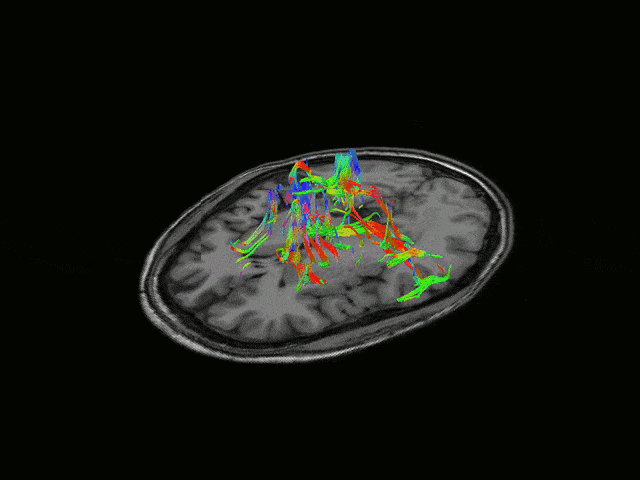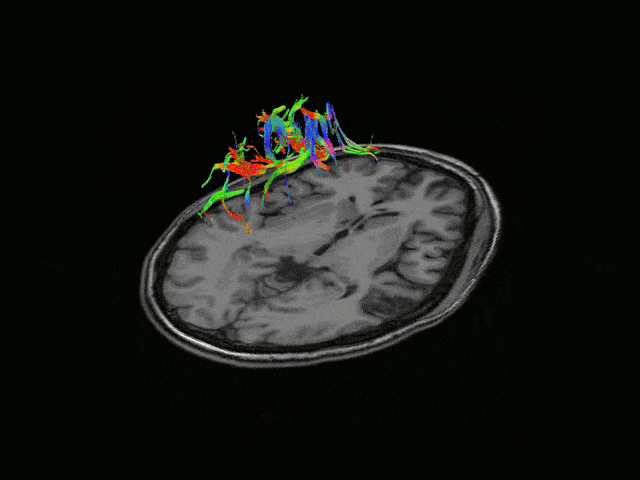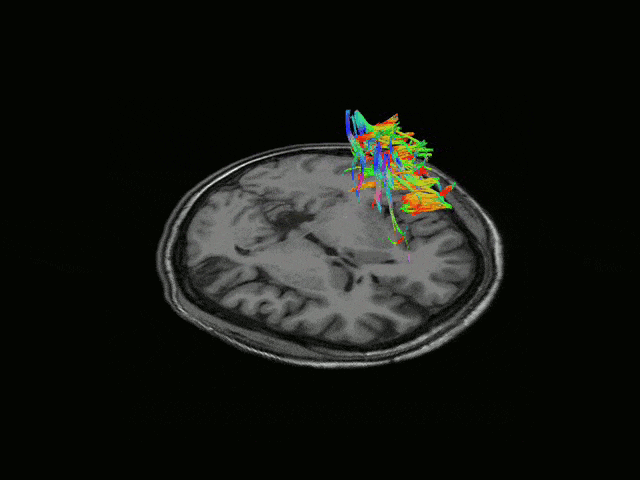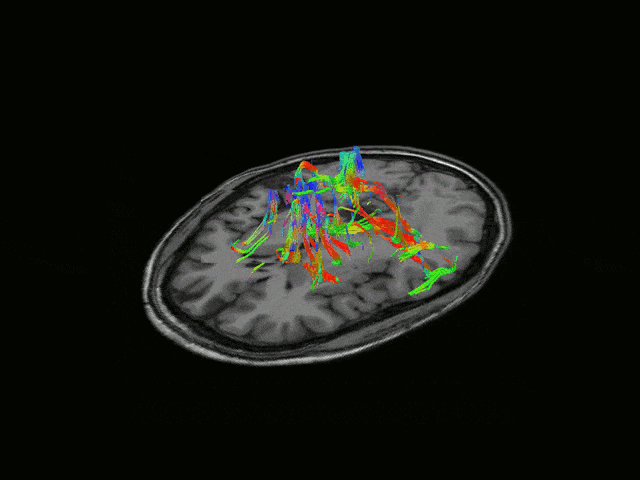
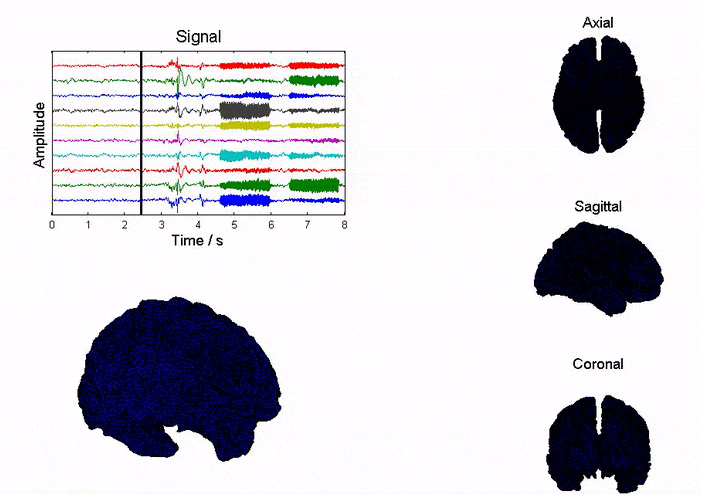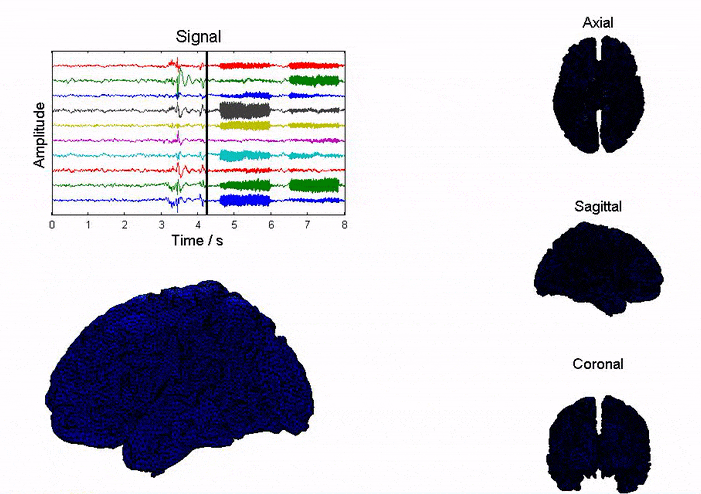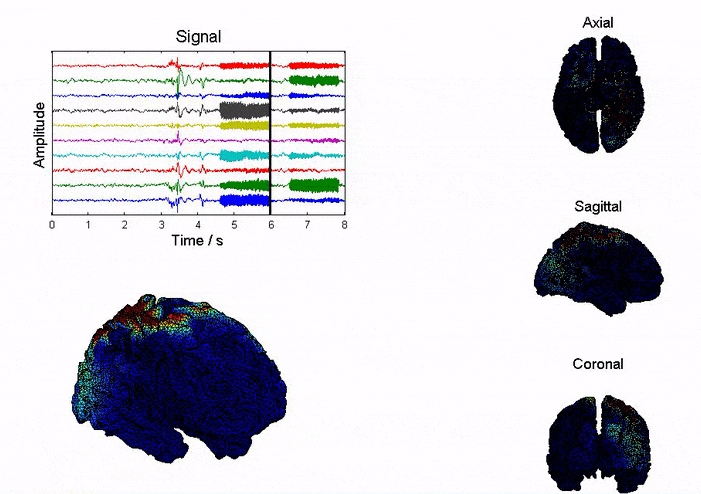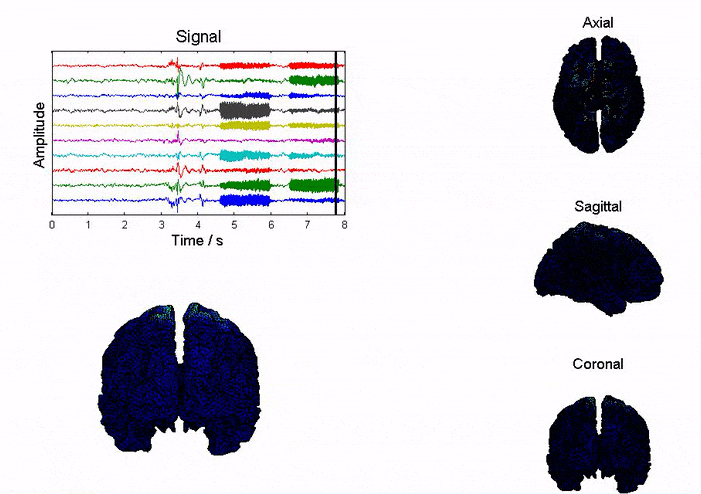
Epilepsy affects ~50 million people globally, and nearly one-third do not respond to medication. Surgical resection has long been an alternative but remains highly invasive with significant risks. Responsive neurostimulation offers a less invasive option, but its lack of specificity limits effectiveness. Adaptive neurostimulation instead responds directly to epileptic features to maximize seizure-stopping capabilities. Recent breakthroughs in patient-specific computational brain modeling enable simulation of brain dynamics and responses to neurostimulation in silico, offering a powerful framework to design and optimize adaptive stimulation strategies before clinical deployment. Individualized models built from neuroimaging and electrophysiological data help identify seizure onset zones, simulate propagation, and test targeted interventions under controlled conditions. This enables precise tuning of parameters, such as timing, amplitude, and location, to maximize seizure suppression and minimize side effects. Compared to traditional approaches, virtual testing accelerates development of safer, more effective, and personalized therapies for epilepsy.
Interpretable AI Powered Prognosis Tool for β-Amyloid Plaque Burden
We developed interpretable machine learning models to predict five-year β-Amyloid (Aβ) plaque progression in individuals at early risk for Alzheimer’s Disease (AD). Focusing on subjects with initially low Aβ burden (Centiloid < 24) from the ADNI cohort, we integrated demographic information, APOE genotype, cognitive assessments, and PET-derived features—including regional SUVRs, brain volumes and their change rates. Using support vector machine, random forest, and multilayer perceptron models, we achieved strong performance (F1-scores up to 0.866), with consistent generalization to the independent OASIS-3 dataset.
To ensure transparency, we applied SHAP (Shapley Additive Explanations) and found that changes in prefrontal SUVR, regional volumes, and SUVRs in parietal and posterior cingulate regions were among the most influential predictors. Our approach offers an interpretable and practical tool for identifying individuals at high risk of Aβ buildup, supporting early diagnosis and timely intervention in AD.

A: Prognosis pipeline for β-Amyloid plaque burden.
B: ML model performance (weighted F1-score).
C: PET feature contributions based on averaged normalized SHAP values from MLP outputs.
Longitudinal analysis on epileptogenesis in Alzheimer’s Disease
Alzheimer’s disease (AD), which accounts for ~70% of dementia cases, shares key mechanisms with late-onset epilepsy, including neuroinflammation and amyloid buildup. Seizures further accelerate Aβ and tau accumulation, worsening cognitive decline, as seen in hAPP-J20 mice.
We conducted a longitudinal study tracking spatial learning, memory loss, and Aβ plaque buildup in Tg and non-Tg mice from birth to 27 weeks. Behavioral tests, immunostaining (NPY, CaMK2, PV), and video-EEG were performed every 3 weeks.
Our results link Aβ deposition to seizure activity, reduced neuronal density, and rising HFOs, especially fast ripples and pathological ripples, highlighting their potential as early biomarkers of epileptogenesis in AD.

Temporal analysis of HFO incidence from chronic CA1 recordings: Ripple (80–200 Hz, top) and fast ripple (250–500 Hz, bottom) rates were similar between nTg and Tg mice before 15 weeks (pre-plaque stage), but showed a significant increase in Tg mice after 17–18 weeks.
Published Paper: https://alz-journals.onlinelibrary.wiley.com/doi/10.1002/alz.087939
Cross Frequency Coupling (CFC)
Cross Frequency Coupling (CFC) is the interaction between brain oscillations of different frequencies, and the coupling phenomenon has been observed in the brain of rodent and human. Phase-amplitude coupling (PAC) is a type of CFC, which describes the dependence between the phase of a low-frequency component and the amplitude of a high-frequency component of electrical brain activities. It has been claimed that the modulation of low frequency phase on high frequency amplitude plays a functional role in cognition and information processing, such as learning and memory. The change of PAC patterns has been associated with various neurological disorders, e.g., epilepsy and schizophrenia.
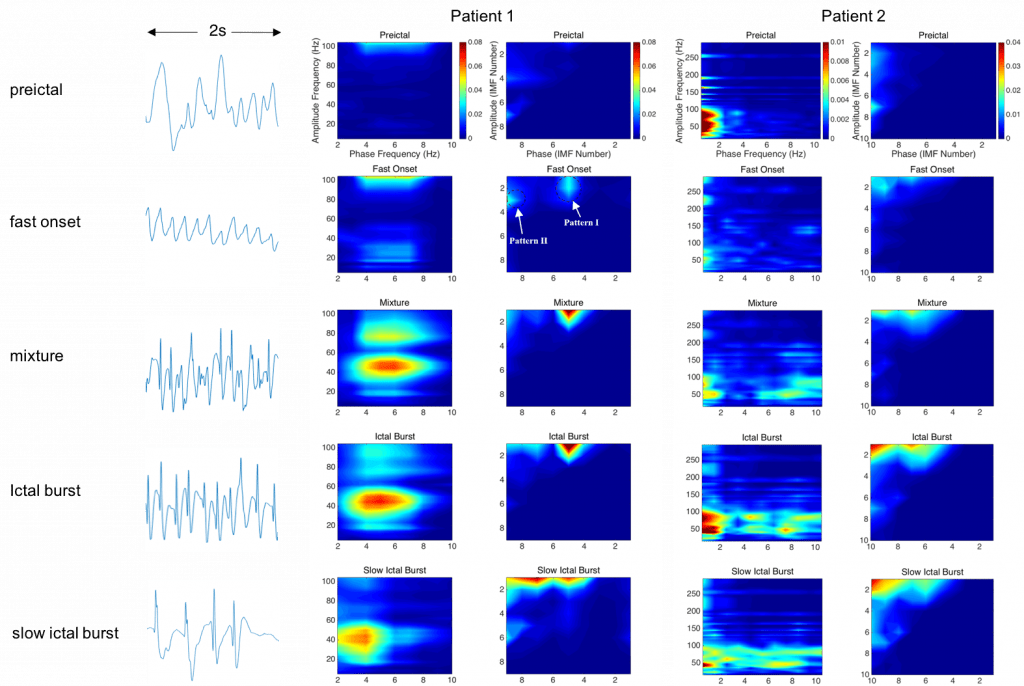
Non-stationary and nonlinear techniques for seizure detection algorithms
Epilepsy is one of the most common neurological diseases, affecting over 3 million people in U.S. and 50 million (~1%) people worldwide. An automated and accurate seizure detection method can be very helpful. Currently, most people use stationary and linear methods (i.e. Fast Fourier Transform (FFT), etc.) to analyze the signal. However, EEG signal is non-stationary and nonlinear in nature, thus these methods will introduce inaccuracy. Hence, we are developing non-stationary and nonlinear algorithms to improve the accuracy of seizure detection.

Collaborators
- Dr. Yue-Loong Hsin, Chung Shan Medical University, Taiwan
Electrode-based Bio-imaging
Our goal is to develop an Electrode-based Bio-imaging System that will be fMRI-competitive. Currently, fMRI is very popular since it offers high spatial resolution (~3mm); however, its temporal resolution is limited (~1s) and is impossible to be used in portable applications. EEG has excellent temporal resolution (~1ms), portable mobility that allowed to be used in daily life. Here, we devote to improving the spatial resolution of EEG in the aspects of both hardware and software.
1. Hardware
We developed a Focused Electrode that improves spatial resolution, recording SNR and reduce crosstalk and correlation with adjacent electrodes. The Focused Electrode is adaptive to the geometry parameters of electrode array including electrode size, pitch and source depth, such as EEG or ECoG. Simulation results show that the Focused Electrode increases the number of electrodes up to 7x in EEG and 30x in ECoG without overlapping information if the array covers half of head.

2. Software
We study the inverse imaging using realistic head model based on MR image (NFT toolbox). On one hand, we are studying the influence of various factors (i.e. noise, electrode number, head model, inverse solution, etc.) on inverse imaging; on the other hand, we are developing more accurate inverse imaging algorithms.
Collaborators
- Prof. Scott Makeig, SCCN, UCSD
- Dr. Yue-Loong Hsin, Chung Shan Medical University, Taiwan
Brain Dynamics and Source Localization
Brain imaging techniques can help us explore the function of human brain (i.e. memory, behavior, etc.) and help diagnosis and treatment of brain disorders (i.e. seizure, depression, etc.). Imaging tool with high temporal and spatial resolution is highly desirable. Currently, fMRI is very popular since it offers high spatial resolution (~3mm); however, its temporal resolution is limited (~1s) and is impossible to be used in portable applications. EEG has excellent temporal resolution (~1ms), portable mobility that allowed to be used in daily life. Our goal here is to improve the spatial resolution of EEG to be as good as that of fMRI.
We study the brain dynamics and source localization based on realistic head model (NFT toolbox). On one hand, we are studying the influence of various factors (i.e. noise, electrode number, head model, inverse solution, etc.) on the accuracy of inverse imaging; on the other hand, we are developing more accurate inverse imaging algorithms.
Collaborators
- Prof. Scott Makeig, SCCN, UCSD
- Dr. Yue-Loong Hsin, Chung Shan Medical University, Taiwan