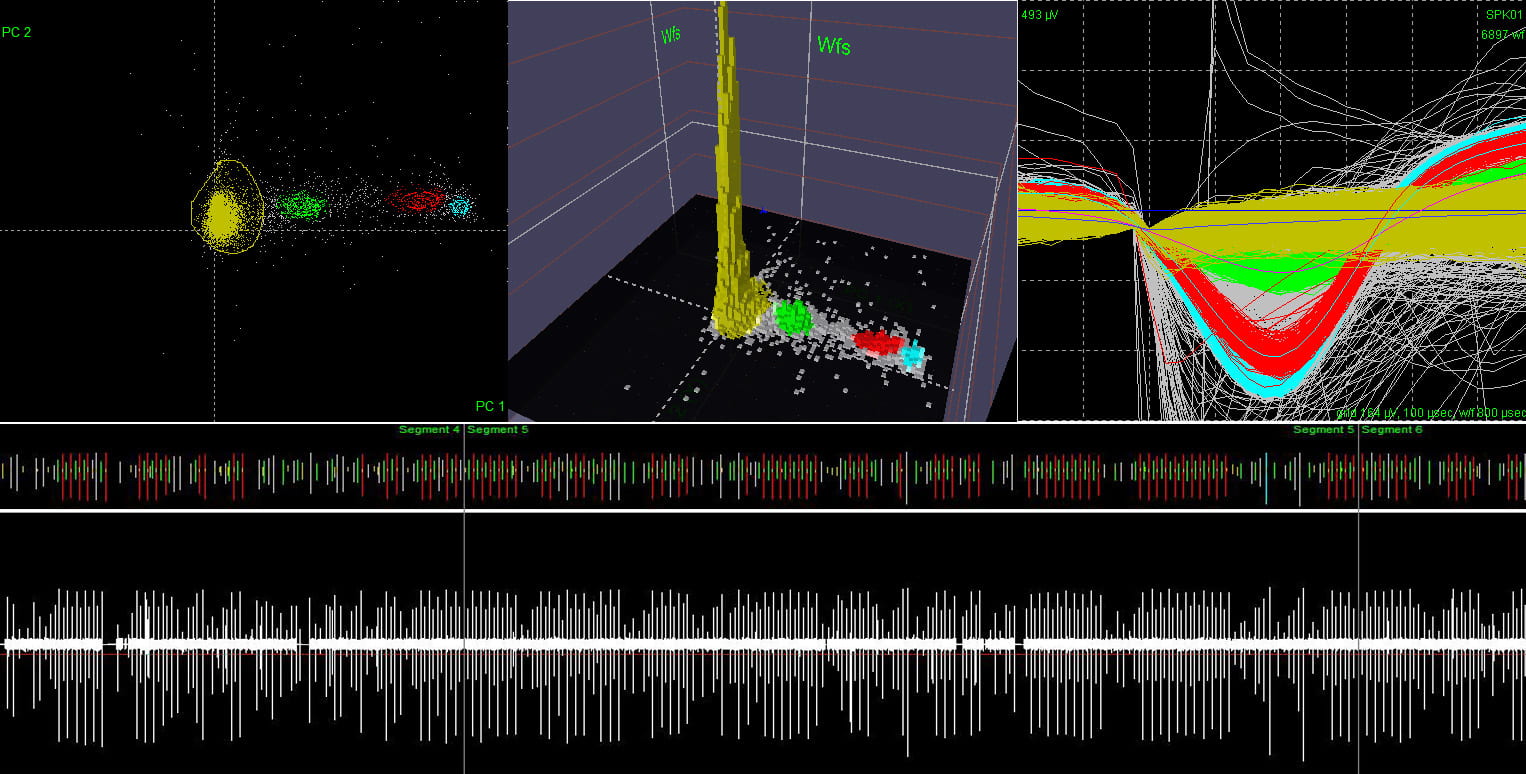
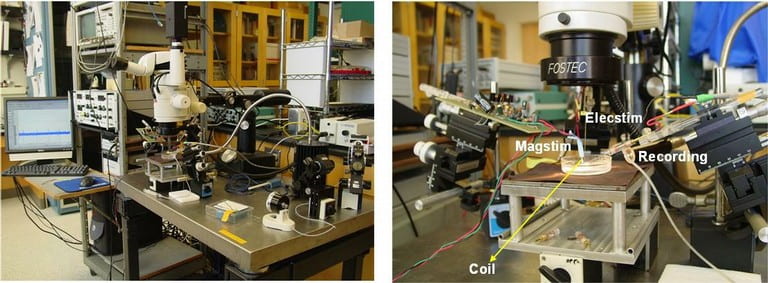
Recording from a large number of neurons produces vast quantities of data that is highly difficult to extract and interpret due to noise and aggregation of multiple neural signals in a single recording site. In addition to conventional techniques like spike sorting, component analysis, de-noising detection, we are focusing on the cellular and molecular levels to understand the fundamentals of neural signals and behavior of large group of neurons. We found that the traditional deterministic channel models are insufficient for the description of the activities of real neurons. We are currently developing the stochastic kinetic modeling of sodium channel and its validation with the measurement of gate current due to transmembrane protein movements in the order of several picoamps with the bandwidth of several hundred of MHz.
Selected Publications
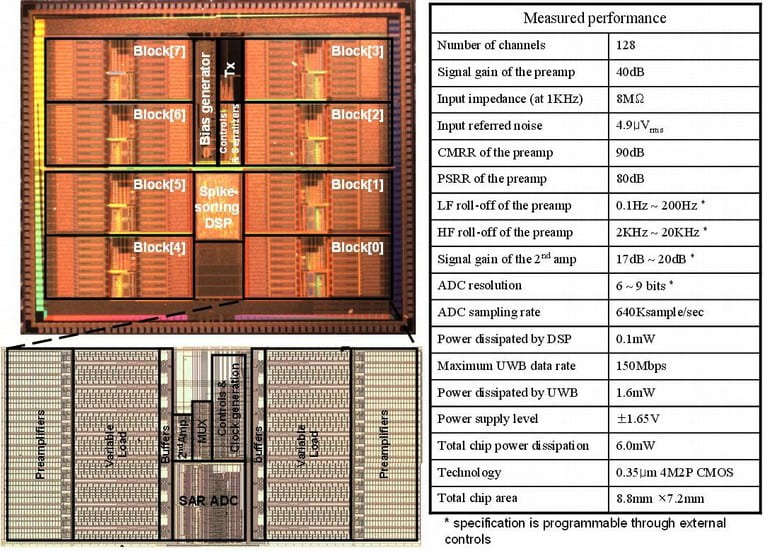
- “A 12-channel 6mW Wireless Neural Recording IC with On-the-fly Spike Sorting and UWB Transmitter,” M. Chae, W. Liu, Z. Yang, T. Chen, J. Kim, M. Sivaprakasam, and M. R. Yuce, To appear in International Solid-State Circuits Conference, February 2008.
- “An Integrated Multi-Channel Neural Recording System,” M. Chae, W. Liu, and M. Sivaprakasam, BMES Annual Fall Meeting, September 2007.
Collaborators
- Huntington Medical Research Institutes
- Arizona State University
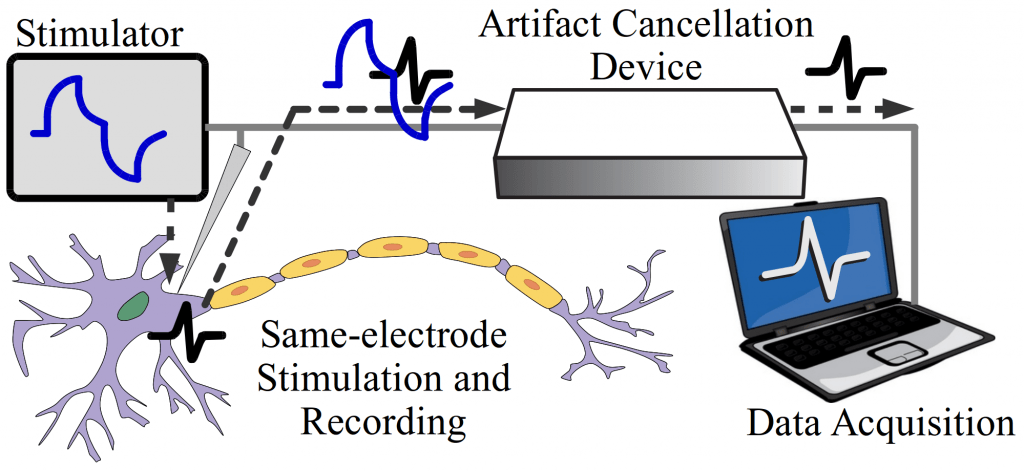
 Live Stimulation Artifact Cancellation
Live Stimulation Artifact Cancellation